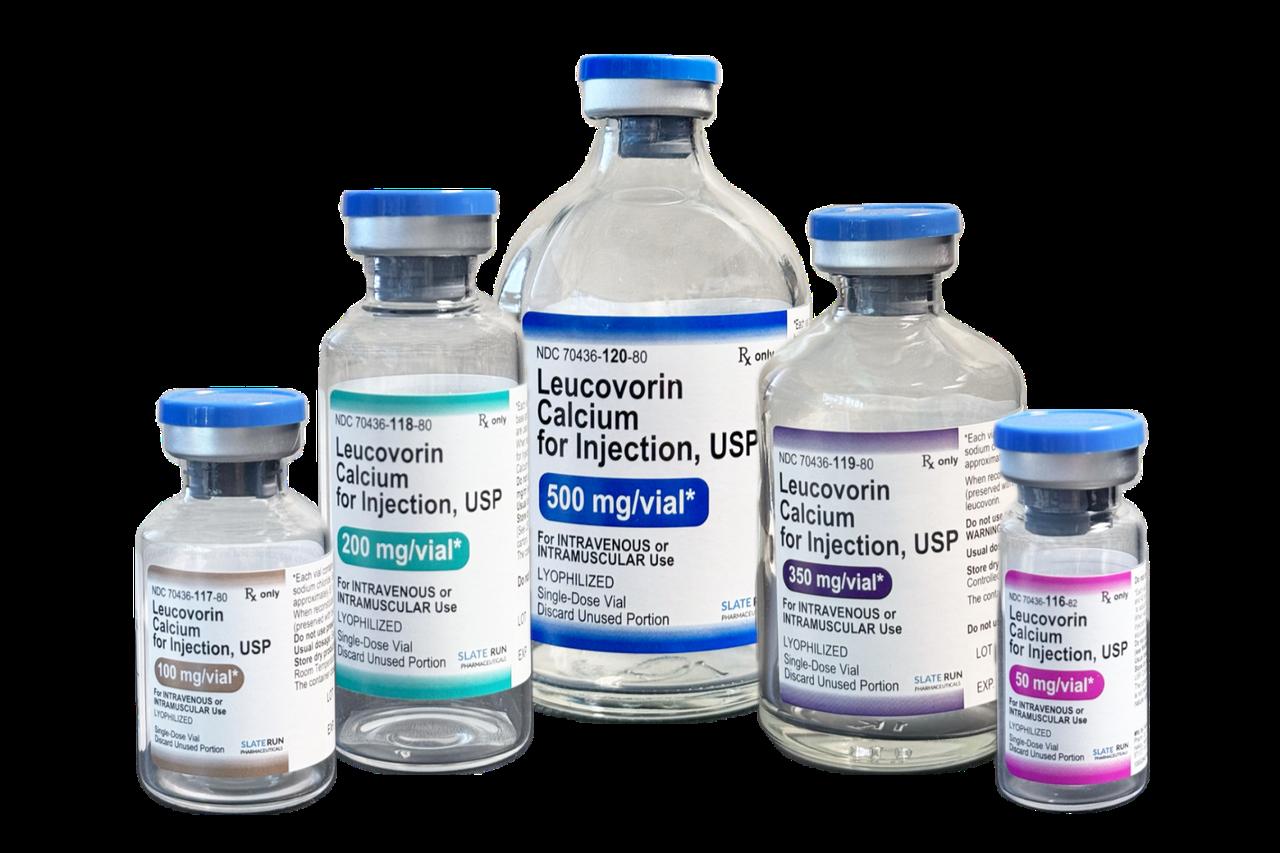
The U.S. Food and Drug Administration approved leucovorin on Monday, a form of folic acid, for treating children with cerebral folate deficiency and autism symptoms, according to federal health officials and regulatory filings.
The approval comes as President Donald Trump was scheduled to highlight the treatment at a White House event Monday, part of broader administration efforts to address autism causes and treatments.
FDA Commissioner Marty Makary, National Institutes of Health Director Jay Bhattacharya and Centers for Medicare and Medicaid Services Administrator Mehmet Oz announced the decision in a joint opinion piece published Monday in POLITICO Magazine.
The officials said leucovorin, also known as folinic acid, showed evidence of helping children with autism improve verbal communication, though they emphasized "it is not a cure for autism."

The FDA published its approval notice in the Federal Register, citing "patient-level data on over 40 patients, including both adults and pediatric patients" supporting the drug's effectiveness for cerebral folate deficiency symptoms.
According to the regulatory filing, cerebral folate deficiency has been documented in patients showing neuropsychiatric symptoms, including autistic features.
Leucovorin, currently used to treat cancer and anemia patients, was previously manufactured by GSK under the brand name Wellcovorin. The company withdrew the drug from market, though not due to safety or effectiveness concerns.
Scientists have expressed cautious optimism about leucovorin's potential for autism treatment while noting the limited scope of current research. The drug, a form of vitamin B, may benefit a specific subset of autism patients who are deficient in folate.
The approval means states will be required to cover leucovorin treatment through Medicaid and CHIP programs, which insure over half of American children, according to the health officials.
The announcement coincides with Health Secretary Robert F. Kennedy Jr.'s April pledge to provide answers about autism causes by September.

The administration officials also addressed acetaminophen use during pregnancy, encouraging "judicious" consultation with healthcare providers amid conflicting research on potential links to autism and ADHD diagnoses.
"Peer-reviewed data from large-scale cohort studies, including the Nurses' Health Study II and the Boston Birth Cohort, find this association," the officials wrote, while acknowledging that "evidence from family control studies have failed to find a correlation."
Medical organizations maintain their current guidance on acetaminophen use. Dr. Peter Bernstein of the American College of Obstetricians and Gynecologists noted that existing studies show associations rather than causation, adding that untreated fevers may pose greater pregnancy risks than acetaminophen use.
The United Kingdom's Medicines and Healthcare products Regulatory Agency reinforced acetaminophen's safety profile Monday, with Chief Safety Officer Alison Cave stating: "There is no evidence that taking paracetamol during pregnancy causes autism in children."